What Is Photoelectric Effect?
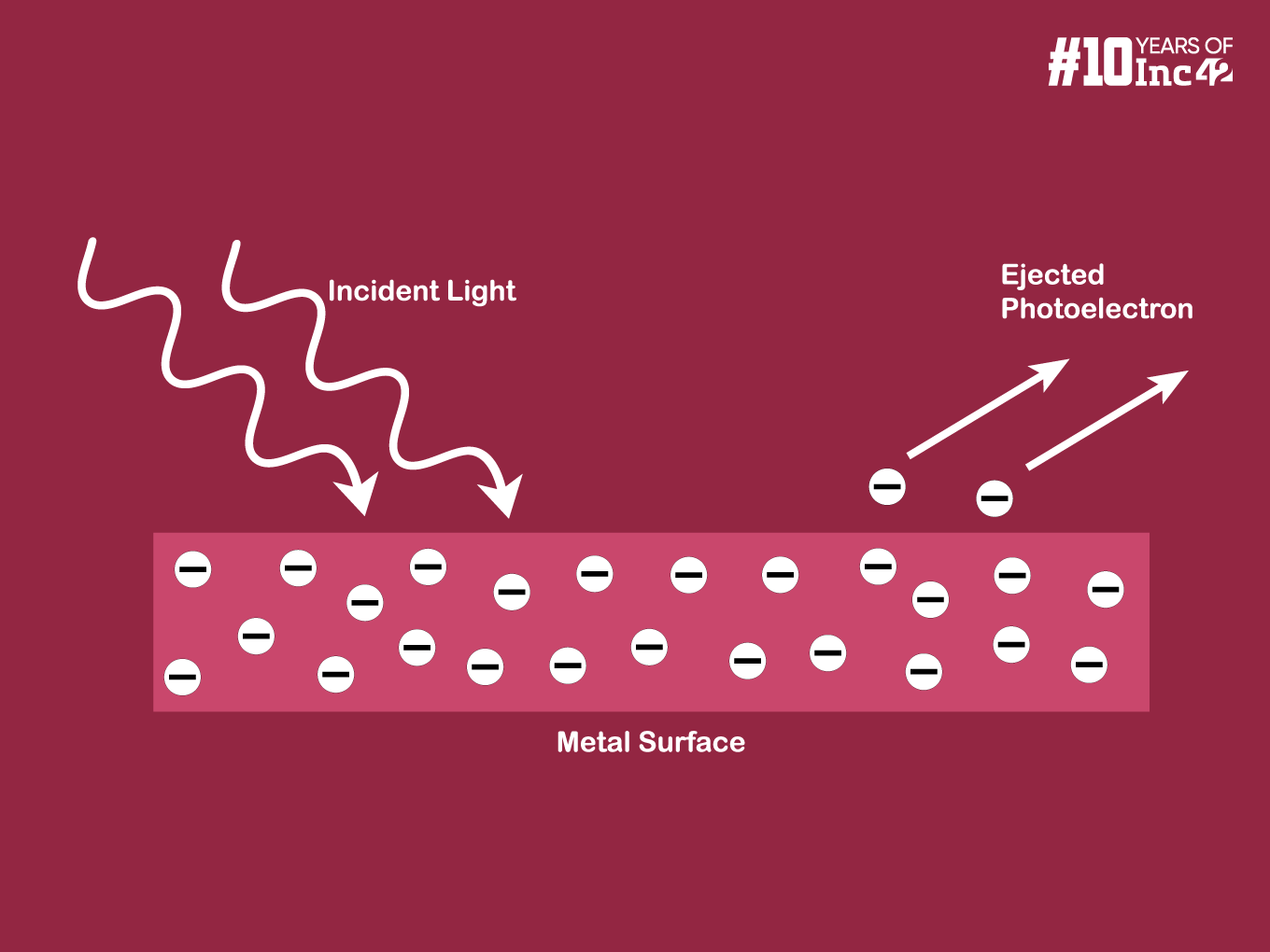
The photoelectric effect is a phenomenon where light knocks electrons out of a material, resulting in the emission of these electrons, called photoelectrons. Albert Einstein explained the photoelectric effect in 1905. He proposed that light comes in packets of energy called photons. These photons bump the electrons with their energy, revolutionising our understanding of light. Einstein received the 1921 Nobel Prize in Physics for his work to explain the photoelectric effect.
How Does The Photoelectric Effect Work?
Consider a metal with bound electrons. Each electron occupies an energy level within the material, and a certain minimum energy, known as the work function (φ), is required to excite the electron to the vacuum level (free space). This work function represents the energy barrier the electron needs to overcome to escape the metal.
When a photon with energy (E) strikes a metal surface, it can interact with a bound electron. This leads to three possible scenarios:
- Inelastic collision (E < φ): If the photon’s energy is less than the work function, it is an inelastic interaction. The photon’s energy is insufficient to overcome the electron’s binding energy. Therefore, no electron emission occurs.
- Photoexcitation (E = φ): When the photon’s energy equals the work function, the electron absorbs the photon’s energy. The electron absorbs the photon and transitions to the vacuum level, escaping the metal with zero kinetic energy (KE = 0).
- Photoemission (E > φ): If the photon’s energy is more the work function, this condition photoemission occurs. The electron absorbs the incident photon and gets ejected from the metal lattice. The ejected photoelectron acquires kinetic energy (KE) according to the following equation:
K=E-
This equation expresses the principle of energy conservation. The total energy gained by the electron is equal to the incident photon’s energy (E) minus the work function (φ) required to escape the metal. The remaining kinetic energy determines the speed of the emitted electron.
Key takeaways from the photoelectric effect:
- Light exhibits particle-like behaviour: The photoelectric effect demonstrates light-matter interaction through quantised energy transfer. The energy exchange between the photon and electron occurs in a single interaction, supporting the particle nature of light (photons) in this context.
- Frequency dependence: Only photons exceeding a threshold frequency, corresponding to the minimum energy required to overcome the work function (f ≥ fthreshold), can eject electrons from the metal surface.
- Intensity Vs. Energy: The intensity, or number of photons, hitting the metal surface influences the number of ejected electrons. However, the individual kinetic energy of each photoelectron is solely determined by the energy of the absorbed photon.
What Are The Applications Of Photoelectric Effect In Semiconductors?
Semiconductors differ from metals in their electronic structure.
Metals have a continuous range of energy levels for electrons, while semiconductors have a bandgap separating two key regions: the valence band, filled with electrons, and the empty conduction band. This bandgap plays a critical role in the photoelectric effect for semiconductors.
Light with energy less than the bandgap energy (Eg) won’t be able to excite electrons across the bandgap in a semiconductor, resulting in no photocurrent. These properties of semiconductors have made the photoelectric effect an important phenomenon for various applications, including:
- Photodetectors: Photodiodes and phototransistors are prime examples. When light strikes a semiconductor, it can generate electron-hole pairs if the photon energy is greater than the bandgap. Electrons move to the conduction band, and holes (the absence of electrons) act like positive charges in the valence band. This flow of charge creates a photocurrent, allowing photodetectors to convert light into electrical signals.
Photodiodes are used in various applications like optical communication receivers, smoke detectors, and image sensors in digital cameras. Phototransistors amplify the photocurrent, making them suitable for light detection in low-light conditions.
- Solar Cells: Solar cells rely on the photoelectric effect in semiconductors to convert sunlight into electricity. When sunlight strikes a solar cell made of silicon, photons with enough energy excite electrons across the bandgap. These free electrons and holes create a current flow, generating electricity. The efficiency of solar cells depends on the material’s bandgap and the ability to absorb sunlight.
- Photoconductive Sensors: Photoconductors are light-sensitive resistors whose conductivity changes with illumination. When light shines on a photoconductor, the generated electron-hole pairs increase the conductivity. This effect allows photoconductive sensors to detect light intensity changes and find applications in light meters, security systems, and automatic lighting control systems.