What Is Photoconductivity?
Discovered by Willoughby Smith in 1873, photoconductivity is the phenomenon where certain materials become more electrically conductive when exposed to light. It is the interplay of light and electricity on a material level.
How Does Photoconductivity Work?
Photoconductivity works by leveraging the interaction of light and electrons in a material, typically a semiconductor. The following is a breakdown of the process:
- Light Absorption: When light shines on the photoconductive material, it carries energy in the form of packets called photons. If a photon has enough energy, it gets absorbed by an electron in the material.
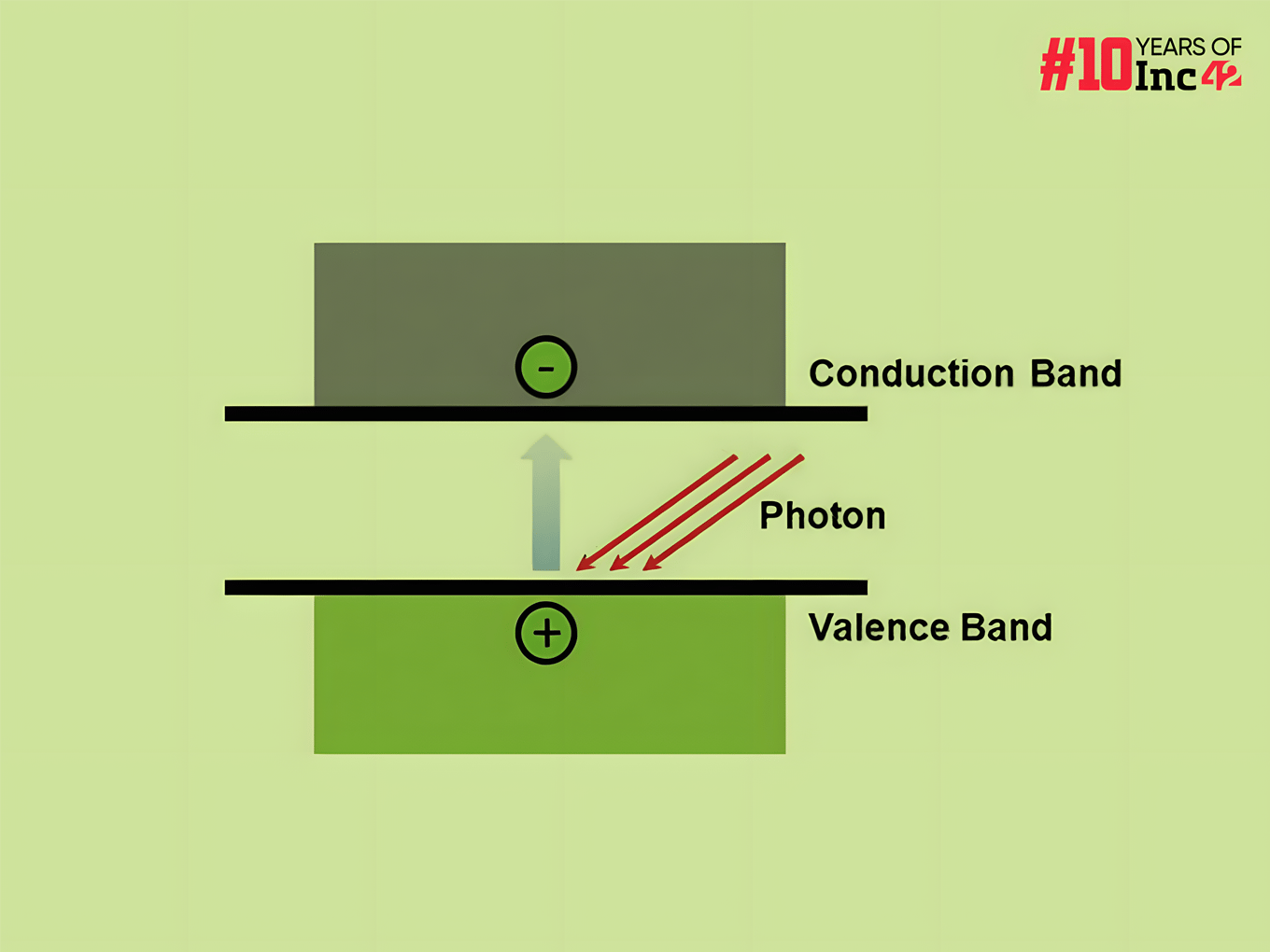
- Band Gap & Excitation: The absorbed energy is crucial. It needs to be greater than the material’s band gap, which is the energy difference between the valence band (where electrons normally reside) and the conduction band (where they can move freely and conduct electricity). If the energy is sufficient, the electron gets excited and jumps across the band gap.
- Generating Charge Carriers: The jump creates two important things: a free electron in the conduction band and a ‘hole’ where the electron was originally in the valence band. This hole behaves like a positive charge carrier since it represents the absence of a negative charge (electron).
- Increased Conductivity: With the free electrons and holes roaming around, the material’s conductivity increases. The more intense the light, the more photons are absorbed, and consequently, more electrons get excited, leading to a larger number of charge carriers and a more significant rise in conductivity.
Various types of electromagnetic radiation can cause photoconductivity, including visible light, ultraviolet light, infrared light, and even gamma radiation. The specific type of effective radiation depends on the material’s properties, particularly its band gap, which is the energy difference between the valence band (where electrons normally reside) and the conduction band (where they can move freely and conduct electricity).
What Are The Applications Of Photoconductivity In Semiconductors?
Photoconductivity in semiconductors plays a vital role in several key technologies:
- Photodetectors: These are devices that convert light signals into electrical signals. They exploit photoconductivity to detect the presence, intensity, or wavelength of light. Here are some examples:
- Light Metres: Photodetectors measure light intensity, making them crucial for photography, scientific experiments, and automatic lighting control systems.
- Smoke Detectors: Certain photodetectors are sensitive to specific wavelengths emitted during combustion, making them ideal for smoke detection in fire alarms.
- Night Vision Goggles: By using photodetectors sensitive to infrared light invisible to the human eye, night vision goggles enable humans to see in low-light conditions.
- Optical Communications: Photodetectors receive light pulses carrying information in fibre optic cables, converting them into electrical signals for processing and transmission.
- Photoresistors (Light-Dependent Resistors or LDRs): These are special resistors whose electrical resistance changes based on the intensity of light they receive. Here’s how they leverage photoconductivity:
- Light-Controlled Circuits: LDRs are used in circuits that automatically adjust based on light levels. For instance, they can control street lamps that turn on as it gets darker or camera light metres that adjust aperture settings.
- Security Systems: LDRs can be part of security systems that detect intrusions by monitoring changes in light levels, potentially triggering alarms.
- Solar Cells: Photoconductivity is a fundamental principle behind how solar cells convert sunlight into electricity. Here’s the process:
- Light Absorption and Charge Carrier Generation: When sunlight strikes a solar cell made from a photoconductive semiconductor (like silicon), photons are absorbed by electrons, causing them to jump to the conduction band, creating electron-hole pairs.
- Current Generation: The solar cell is designed with an electric field that pushes these free electrons in one direction and holes in the opposite direction, creating an electric current.
- Electricity Production: This current can be used to power devices or fed into a grid for wider electricity distribution.