What Is Bandgap?
Also referred to as an energy gap, bandgap is a concept in solid-state physics and chemistry. It describes the region of an element’s crystal’s electronic band structure through which electrons can’t pass. In layman’s terms, it is the energy range within a material unsuitable for conduction of electric current.
Imagine the allowed energy levels for electrons in a solid material are like quantised energy levels in atoms. In a crystal, these atomic energy levels broaden into bands separated by forbidden energy gaps due to the periodic arrangement of atoms.
The bandgap is the energy difference between the top of the valence band, which consists of tightly bound electrons in atomic orbitals, and the bottom of the conduction band, which contains mobile electrons that can form an electric current.
How Does Bandgap Impact Conductivity?
The bandgap dictates the conductivity of a material. For electricity to flow, a material needs mobile electrons that can carry the current. These mobile electrons reside in the conduction band. The bandgap acts as an energy barrier between the valence band (filled with electrons) and the conduction band (where conduction happens).
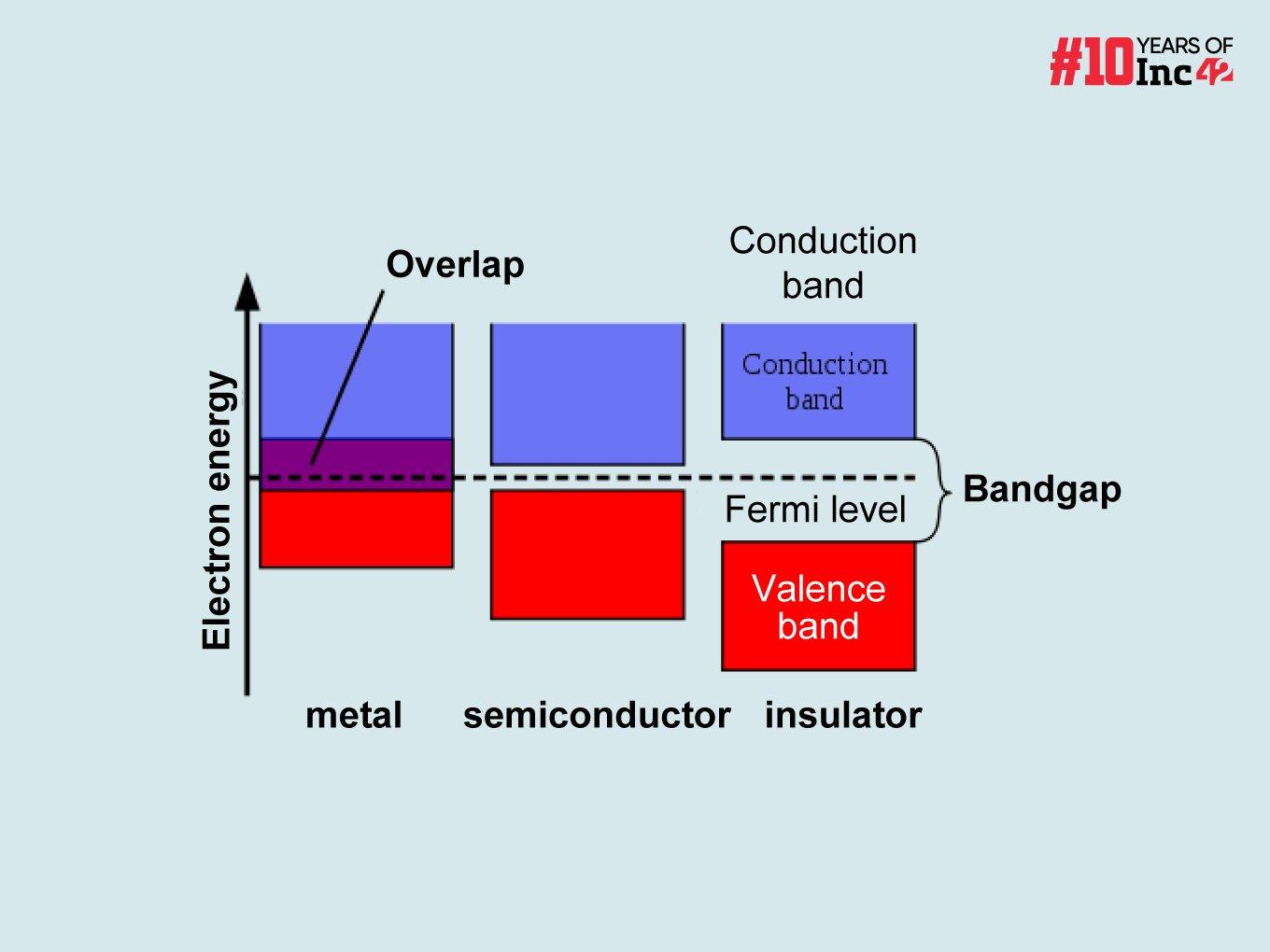
There are three possible states, which define the three types of materials based on their conductivity: insulators, semiconductors/semi-metals and conductors.
- Large Bandgap = Poor Conductivity: In insulators, this bandgap is large. This means a significant amount of energy is needed to excite an electron across the gap and into the conduction band. At normal temperatures, thermal energy is not enough to overcome this barrier. So, very few electrons become mobile, resulting in low conductivity.
- Small Bandgap = Potential For Conductivity: Semiconductors have a smaller bandgap. With enough energy, either through heat or light (via the photoelectric effect), an electron can jump the gap and become mobile in the conduction band. This allows some current to flow, making them useful for controlling electrical signals.
- Very Small/No Bandgap = Good Conductors: In conductors, the bandgap is very small or nonexistent. The valence and conduction bands overlap, so electrons can move freely between them. This abundance of mobile electrons makes conductors excellent at carrying electricity.
The bandgap is not the only factor affecting conductivity, but it is a fundamental concept. By understanding the bandgap and how it influences electron movement, we can explain the behaviour of different materials and develop new technologies with tailored electrical properties.
What Is The Role Of Bandgap In Semiconductors?
In terms of bandgap, semiconductors sit in a sweet spot that makes them extremely useful for applications that involve fine control over electron movement. They have a moderate bandgap, typically ranging from 0.1 to 1.39 electronvolts (eV).
This bandgap is large enough to prevent them from conducting like metals at room temperature, but small enough for them to be influenced by external factors. The key to manipulating semiconductors lies in their responsiveness to external energy sources. This energy can come in three main forms:
- Heat: At higher temperatures, some electrons in the valence band gain enough thermal energy to jump the bandgap and reach the conduction band. This creates a small number of mobile electrons, allowing for some conductivity. This is why the conductivity of semiconductors increases with temperature, unlike metals.
- Light (Photons): When light shines on a semiconductor with enough energy (photons exceeding the bandgap), it can excite an electron across the gap. Called the photoelectric effect, this process is crucial for applications like solar cells and LEDs.
- Potential Voltage: When a potential difference is applied across a semiconductor material, it allows electrons to break free of the valence band and join the conduction band. With doping, a finer control can be achieved, which makes electronic components like transistors possible.
Bandgap Engineering & The Future Of Semiconductors
Bandgap engineering is a technique for manipulating the bandgap of a material, specifically semiconductors. It is essentially fine-tuning a material’s response to electricity and light. Bandgap engineering allows scientists to modify this inherent bandgap in various ways:
- Alloys & Layered Structures: By creating alloys of different semiconductors or building layered structures with alternating compositions, the bandgap can be adjusted. Imagine combining paints with slightly different colours to create a new specific shade.
- Strain: Applying controlled strain, either through epitaxial growth (growing thin films) or other methods, can subtly alter the bandgap. Think of stretching a rubber band – it changes its properties slightly.
By engineering the bandgap, scientists can create semiconductors with specific properties for a wide range of applications:
- Light-Emitting Devices (LEDs): By precisely tuning the bandgap, engineers can design LEDs that emit specific colours of light, crucial for displays and solid-state lighting.
- Solar Cells: Tailoring the bandgap allows for more efficient absorption of specific wavelengths of light, improving solar cell efficiency in converting sunlight to electricity.
- High-Speed Electronics: Bandgap engineering can create semiconductors that operate faster and at lower power consumption, enabling the development of next-generation transistors and integrated circuits.
- Novel Materials: The ability to engineer bandgaps opens doors to exploring entirely new materials with exotic properties, potentially leading to breakthroughs in areas like spintronics and quantum computing.